What Are Exoplanets And How NASA Detects Life Beyond Our Solar System
Bharti Airtel Set To Acquire Telenor India Within This Year
Google Celebrates NASA’s Discovery Of Seven Earth-Like Planets With An Animated Doodle
Some Home Remedies That Might Sound Bizarre But Actually Work Like A Charm
Akshay Kumar Feels He Has Made Enough Money, Now Wants To Focus On Content & Characters
Delhi ATM Dispenses Fake Rs 2000 Notes From ‘Childrens Bank of India’ With ‘Churan Lable’
Adolf Hitler’s Personal Telephone During World War II Is Up For Auction In The US
From Salman Khan To Rekha, Neil Nitin Mukesh’s Wedding Reception Was Quite A Starry Affair
FDA Approves ‘Female Viagra’ Pill Flibanserin After Two Rejections
The first drug to treat low sexual desire in women won approval from US health regulators on Tuesday, August 18, but with a warning about potentially dangerous low blood pressure and fainting side effects, especially when taken with alcohol.
The US Food and Drug Administration said the pink pill, to be sold under the brand name Addyi, and made by privately held Sprout Pharmaceuticals, will only be available through certified and specially trained health care professionals and pharmacies due to its safety issues.
If Addyi is consumed with alcohol it is likely to have side effects | Source: Mummywifewoman, Reuters
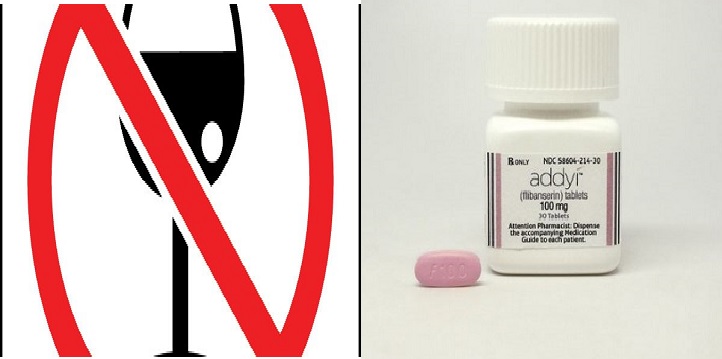
Addyi, whose chemical name is flibanserin, is designed for premenopausal women whose lack of sexual desire causes distress. The condition is formally known as hypoactive sexual desire disorder, or HSDD. The drug needs to be taken daily.
Addyi has been nicknamed the 'female Viagra' even though it does not work like Pfizer Inc's blockbuster Viagra pill for men that in 1998 became the first approved drug for erectile dysfunction.
"This is the biggest breakthrough in women's sexual health since the advent of 'the Pill' for contraception," The National Consumers League said in a statement. "It validates (and) legitimizes female sexuality as an important component of health."