What Are Exoplanets And How NASA Detects Life Beyond Our Solar System
Bharti Airtel Set To Acquire Telenor India Within This Year
Google Celebrates NASA’s Discovery Of Seven Earth-Like Planets With An Animated Doodle
Some Home Remedies That Might Sound Bizarre But Actually Work Like A Charm
Akshay Kumar Feels He Has Made Enough Money, Now Wants To Focus On Content & Characters
Delhi ATM Dispenses Fake Rs 2000 Notes From ‘Childrens Bank of India’ With ‘Churan Lable’
Adolf Hitler’s Personal Telephone During World War II Is Up For Auction In The US
From Salman Khan To Rekha, Neil Nitin Mukesh’s Wedding Reception Was Quite A Starry Affair
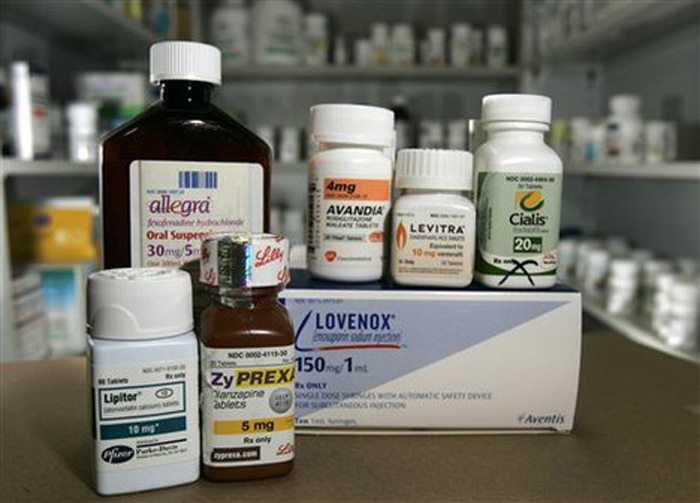
Former Promoters Of Pharma Company Ranbaxy Fined Rs 2,500 Crore By A Singapore Court
Erstwhile Ranbaxy promoters, Malvinder and Shivinder Mohan Singh, will challenge a Singapore court's recent order which has imposed a fine of $400 million (over Rs 2,500 crore) for allegedly concealing and misrepresenting facts from Japanese pharma firm Daiichi Sankyo when they had sold the promoter's stake to it for $2.4 billion in 2008. In a case slapped three years ago by the Japanese company against the Singh brothers for allegedly hiding information regarding Ranbaxy's FDA troubles, a three-member bench of the Singapore Court of Arbitration recently issued an award by 2:1 in favour of Daiichi. In the legal suit, the Japanese company had accused the erstwhile Ranbaxy promoters of concealing and misrepresenting facts at the time of its $2.4 billion purchase of a controlling stake (34.8%) in the company in 2008.
"It is going to be a long drawn legal dispute, and will be challenged," sources close to the Singh family told TOI. The Singh family is exploring "further legal options to challenge" court verdict. A statement said "in an arbitration dispute between Daiichi Sankyo and the sellers of shares of erstwhile Ranbaxy Laboratories, which includes RHC Holding and Oscar Investments as a party, the Arbitration Tribunal has issued an award, where the law governing dispute was Indian law by a majority of 2:1 in favour of the claimant (Daiichi)."
It adds Justice A M Ahmadi, former Chief Justice of India, gave a dissenting opinion dismissing all Daiichi claims for damages of Rs 2,562 crore, quantified interest, costs and expenses of the arbitration.
Oscar Investments promoted by the Singh family, lost 4% on BSE to close at Rs 222, while group company Fortis Healthcare lost nearly 2% to close at Rs 170. While BSE has sought clarification from Oscar Investments, it is not known at this point whether Sebi will investigate the case. In 2013, the Japanese company had sought compensation for losses that it was forced to pay the US Department of Justice.

Soon after it was acquired by Daiichi, Ranbaxy's troubles started when the US drug regulator slapped warning letters on its key manufacturing facilities at Paonta Sahib (Himachal Pradesh) and Dewas (Madhya Pradesh), while in the following year it banned the import of medicines due to manufacturing lapses.
In May 2013, Ranbaxy under the management control of Daiichi was forced to reach a $500-million settlement with the US Department of Justice that the company manipulated test results to get the USFDA approval for its medicines.
Bogged down by US court cases, regulatory woes and losses, Daiichi, that had spent over $4 billion to acquire nearly 60% in Ranbaxy, finally decided to exit from the company. In 2014, Sun Pharma agreed to buy the company from Daiichi in a deal valued at $4 billion, including debt.